 News
News
Echo™-Endothelial
No-fear scaling of endothelial cells
Delivers characterized endothelial cells at clinical scales through a fully GMP-ready workflow.
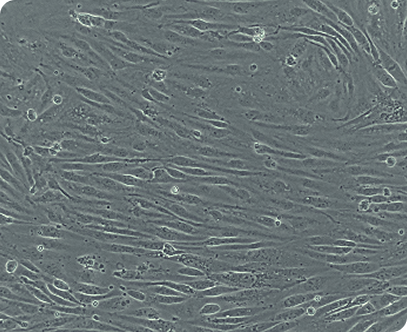
Overcoming the challenges of scaling endothelial cells.
Manufacturing endothelial cells is notoriously hard to scale, with many research-grade protocols delivering low-to-moderate purity and requiring enrichment steps that don’t translate into GMP production.
Echo™-Endothelial, available only from Cellistic, solves this by delivering >90% lineage specificity through a fully GMP-ready manufacturing platform, unlocking the therapeutic potential of endothelial cells across vascularization-driven applications.
By integrating a pre-established iPSC-to-endothelial differentiation process with scalable GMP manufacturing and a complete CMC framework, Echo™-Endothelial provides developers with predictable performance, consistent endothelial function, and regulatory alignment—reducing development risk and enabling a confident path toward IND/IMPD submission.
Accelerate speed to clinic through a pre-established, iPSC-based GMP manufacturing platform
Reduce process and analytical development challenges with pre-built platform and pre-established starting materials
Boost scalability using stirred-tank bioreactors
Secure clear IP positioning and licensing framework
Ensure regulatory readiness and GMP compliance at our EMA-certified, and FDA and PMDA-compliant manufacturing facility
Work with a proven endothelial manufacturing partner delivering consistent >90% purity across batches — without purification steps
How to succeed in endothelial cell manufacturing.
Endothelial cells can show a clear stubborn streak when pushed to scale. That’s where our Echo™-Endothelial platform – and our expertise in how endothelial cells function – come in. Echo™-Endothelial integrates iPSC manufacturing expertise with standardized GMP workflows to ensure scalability, reproducibility, and regulatory alignment for your endothelial program.
Qualified iPSC starting materials
Manufacturing starts from pre-established GMP iPSC master cell banks (MCBs).- Genomic stability, low-passage lines, and full traceability
- Long-term supply security
- Optional integration of client-specific edits via Pulse™ and STAR-CRISPR™
Scalable 3D bioreactor expansion
- Controlled, reproducible expansion in automated stirred-tank bioreactors
- Clinical-scale output exceeding 20B cells per batch
- Consistent viability, purity, and phenotype maintained across scale
Standardized, GMP-aligned differentiation
- Cytokine-free, chemically defined workflows
- Line-agnostic design supporting client-supplied iPSC lines
- Closed, automated systems minimizing variability
- Pre-established batch records and qualified GMP raw materials

Give your endothelial program the start, middle and finish it deserves.
The Echo™-Endothelial platform might be exactly the manufacturing ally to advance your endothelial program on the best possible trajectory – and at the fastest speed – from beginning to end. So why wait to put it to work?

Everything you need to advance efficiently and effectively.
Echo™-Endothelial produces a full suite of GMP-grade endothelial cell materials and documentation packages ready for translational and clinical advancement.
GMP-manufactured endothelial cells
Delivered at scales suitable for Phase 1 clinical studiesComprehensive documentation package
- Certificates of Analysis
- Full traceability logs
- Batch records
- QC release panel
- Regulatory-ready CMC documentation
IP under one roof
Direct access to STAR-CRISPR™, Allo Chassis™, and Echo™ manufacturing with no third-party entanglements
Consistent, reproducible output
- Functionally relevant and physiologically reflective of in vivo endothelial biology
- Reproducible with tight biological variability control
- Fully cGMP compliant, suitable for in vitro modeling, screening, and clinical-scale manufacturing
See how we're leading cell therapy manufacturing.
View all
The platform that delivers momentum from the word “Go!”
When you trust Cellistic and the Echo™-Endothelial platform to push your endothelial manufacturing program forward, you unleash a system purpose-built to advance iPSC-based cell therapy and regenerative medicine programs. Let’s see if Echo™-Endothelial is a good match for you.

