Speed to clinic
Long and unpredictable development timelines, 24months+.
Standardized iPSC-to-NK workflows enable predictable, accelerated timelines. No process or analytical development is required, only 7 months for process adaptation, the entire offering in 18 months.
Difficulty forecasting costs due to process variability.
Fixed pricing model for accurate and reliable cost forecasting.
Achieving sufficient yields for long-term supply security.
Scalable, high-yield Echo™-NK process delivering consistent, off the shelf supply.
Advancing with confidence in a comparatively new regulatory environment.
Your NK drug product is produced in our purpose-built EMA-certified, FDA compliant facility.
Speed to clinic
Long and unpredictable development timelines, 24months+.
Standardized iPSC-to-NK workflows enable predictable, accelerated timelines. No process or analytical development is required, only 7 months for process adaptation, the entire offering in 18 months.
Difficulty forecasting costs due to process variability.
Fixed pricing model for accurate and reliable cost forecasting.
Achieving sufficient yields for long-term supply security.
Scalable, high-yield Echo™-NK process delivering consistent, off the shelf supply.
Advancing with confidence in a comparatively new regulatory environment.
Your NK drug product is produced in our purpose-built EMA-certified, FDA compliant facility.
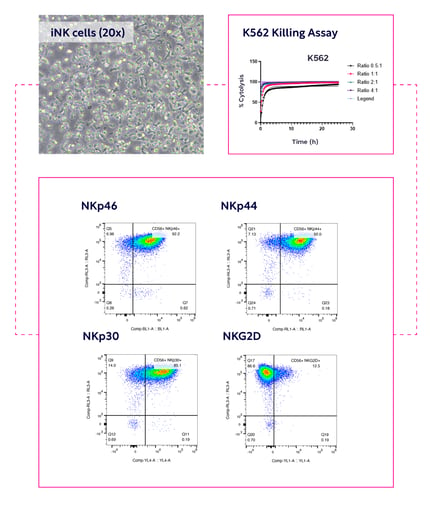
Discover what comes standard with the Echo™-NK offering
Key elements of our Core Offering — delivering 20+ billion cells in 18 months for €3 million — include:
- End-to-end platform process — client provides their unique CAR construct(s)
- Scalable iPSC-to-NK expansion (30×) with bioreactors up to 50–100 L, from early to commercial scale
- Up to 200 doses per batch
- Feeder-free process with fully defined, GMP-compliant materials
- Robust, qualified vendor supply chain
Download the full Core Offering Overview to see everything that’s included.
Echo-NK Platform Overview & In-Process Controls
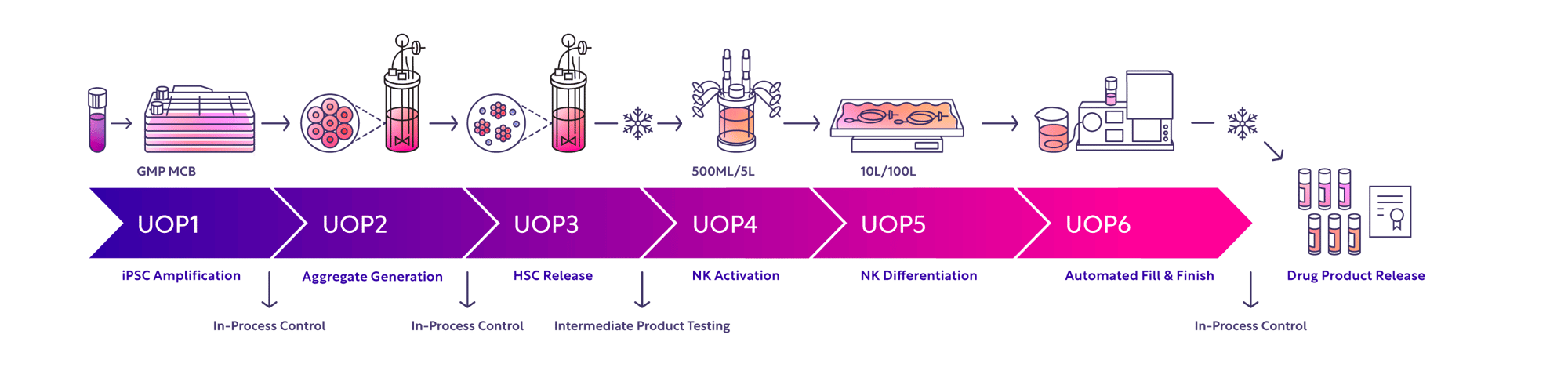
A process built for quality and consistency in iPSC-NK manufacturing
Generating clinical-grade iPSC NK cells at consistent yields with Echo-NK is made possible through a proven process built in unit operations (with multiple in-process controls) and through multiple cryopreservation steps.GMP Facility for iPSC-Derived Allogeneic Cell Therapies

In Mont-Saint-Guibert, Belgium, you’ll find our headquarters – and the world’s first facility dedicated exclusively to iPSC-based cell therapy manufacturing. It’s an agile, flexible, 20,000+ sq. ft. facility that incorporates leading-edge technologies. All materials and operations are validated according with cGMP standards, and manufacturing is conducted in an EMA-certified, FDA compliant GMP facility.
Grade B
Manufacturing Suites
Grade C
Manufacturing Suites
Clean Rooms for
CLD/GMP Manufacturing
GTP Inspections
GMP Inspections
Allogeneic Batches Released

Save time through an 80% pre-developed framework and, where needed, product-specific customization.

Ensure reproducibility, simplify IP management, and provide long-term supply security though renewable GMP iPSC banks and STAR-CRISPR™ editing.

Inform release criteria and comparability protocols through assays that test for identity, safety, and purity.

Scale confidently – and in regulatory alignment – through Pulse™ automation, 3D bioreactors, and processes that can yield up to 100L using the same technologies.

Speed trial starts and positively impact company valuation through access to clinic-ready material in 18 months (versus industry-standard 24-month timelines).

Capitalize on scalable production process founded on iPSC cell lines that eliminate the need of expensive component editing at a manufacturing scale (i.e., viral vectors/DNA/RNA).

Improve margins, market penetration, and long-term program sustainability by leveraging favorable per-dose COGS, scalable production, and a broader patient reach.

Enhance competitiveness, investor confidence, and program longevity by adopting a model purpose-built to meet the needs of NK cell therapy innovators.
Scientific Advantages

Save time through an 80% pre-developed framework and, where needed, product-specific customization.

Ensure reproducibility, simplify IP management, and provide long-term supply security though renewable GMP iPSC banks and STAR-CRISPR™ editing.

Inform release criteria and comparability protocols through assays that test for identity, safety, and purity.

Scale confidently – and in regulatory alignment – through Pulse™ automation, 3D bioreactors, and processes that can yield up to 100L using the same technologies.
Commercial Advantages

Speed trial starts and positively impact company valuation through access to clinic-ready material in 18 months (versus industry-standard 24-month timelines).

Capitalize on scalable production process founded on iPSC cell lines that eliminate the need of expensive component editing at a manufacturing scale (i.e., viral vectors/DNA/RNA).

Improve margins, market penetration, and long-term program sustainability by leveraging favorable per-dose COGS, scalable production, and a broader patient reach.

Enhance competitiveness, investor confidence, and program longevity by adopting a model purpose-built to meet the needs of NK cell therapy innovators.
FAQ
The Echo™-NK offering is ideal for clients taking an iPSC-derived NK cell therapy to Phase I clinical trials.
Doses are therapy-specific. We guarantee a yield of 20+ billion cells, with doses determined by your distinct product and dosage needs.
Echo-NK includes use of Cellistic cell lines (unedited or from our Allo Chassis™ lines). If you prefer to use your own cell line, we can evaluate that option together.
Yes. The offering includes access to these technologies under a licensing framework with milestone payments tied to the achievement of certain clinical and regulatory milestones.— there are no upfront costs.
Our Echo™-NK offering is optimized for our proprietary STAR-CRISPR™ technology to deliver the fastest path to clinic. In addition, our team has broad expertise in other gene-editing technologies and can develop a tailored proposal to fit your needs.
No third-party licenses are required.
Cellistic’s program was designed to achieve 20 billions cells or more via several strategies to scale up or out, if required. Before GMP manufacturing, feasibility runs are performed to confirm performance and de-risk the process. If adjustments are needed, they are addressed prior to initiating a GMP run.
Stability studies are not included in the core offering, but they can be added as an optional service depending on your needs. This approach keeps the base offering cost-competitive while allowing flexibility through à la carte add-ons.
The Echo™-NK core offering supports Phase 1, but based on how we designed our GMP facility, platforms, and processes, Cellistic is able to transition to late phase/pivotal manufacturing with our clients. The current facility is EMA-certified and EU/FDA-compliant.
The current Echo™-NK offering includes one round of multiplex editing (up to three edits). If you wish to introduce additional edits, we can evaluate and incorporate them as a customized à la carte option.
Yes, we have in-house and outsourced analytical methods.
Yes, you can tech transfer your own analytical methods. We will work with you to evaluate and implement this as a customized option.
To learn more about expected pass-through costs, simply reach out to our BD team using the form below. They’ll be happy to help!
The Client and Cellistic will enter into a 1) Master Service Agreement (MSA), 2) Quality/Technical Agreement (QTA), and 3) licensing agreement to define the framework on how technology can be accessed and applied. Non-GMP services can begin under a Letter of Intent (LOI) while these agreements are finalized.
Day of signature. We can also kickstart non-GMP activities under a Letter of Intent while the parties negotiate the Manufacturing Services Agreement (MSA).
Based on extensive experience with our platform and assuming 1) client’s gene edits do not impact NK cell viability or function and 2) the client’s CAR construct is ready to ship to upon contract execution, we will deliver drug product in 18 month timeline .
Discover what comes standard
with every Echo-NK package
Behind every Echo-NK package is a team of experts and a suite of capabilities that are primed to speed your NK program to clinic. Download our Package Overview for a more detailed look at the technologies, services, documentation, and testing that make Echo-NK so unique. 
Learn more about what Echo-NK can do for you
If accelerating your NK program efficiently is a critical priority for your team, then you'll find our team at Cellistic to be an ideal partner. No matter your starting point, our expertise can help you advance to clinic with speed, scalability, and cost efficiency.



