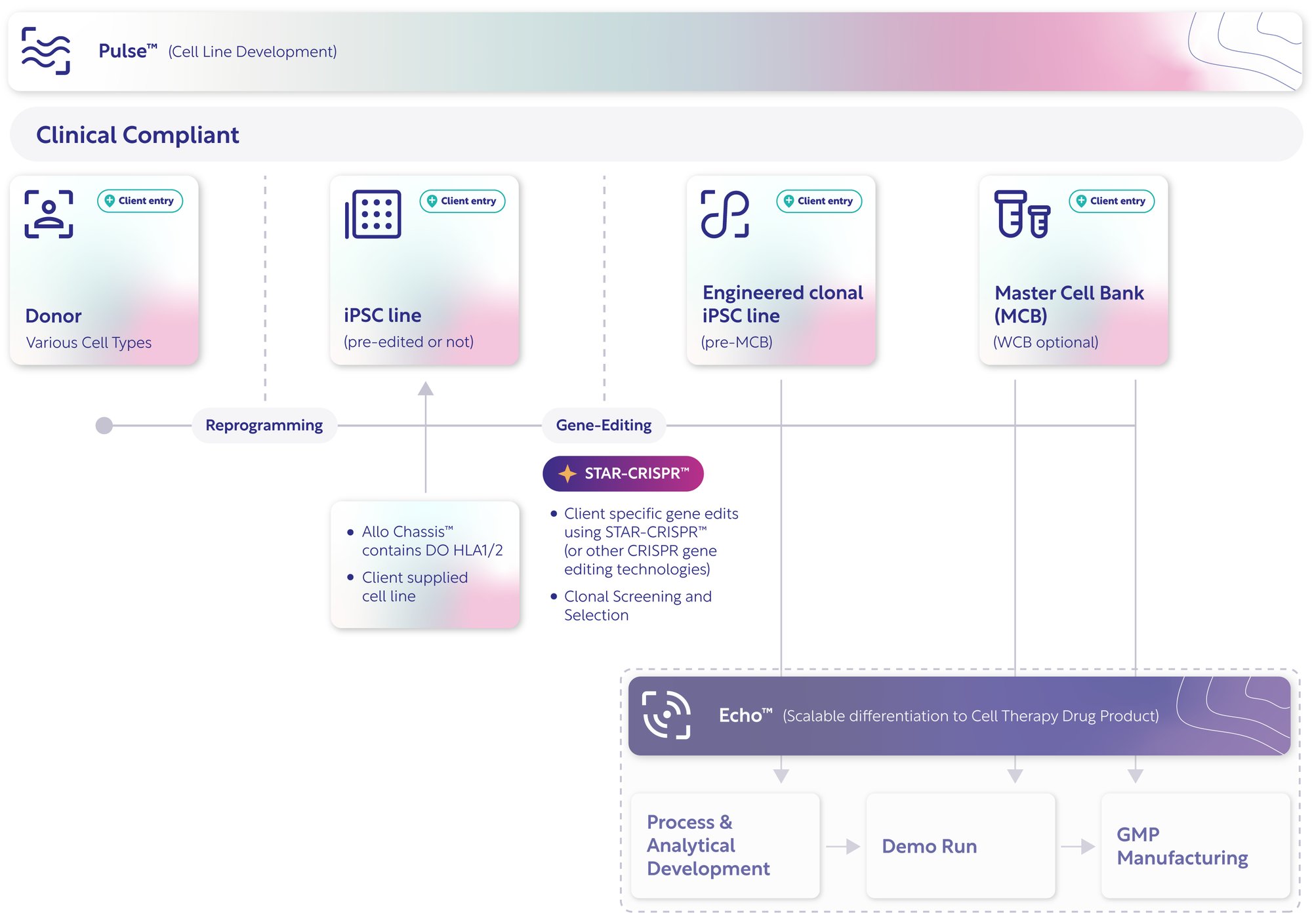
Pulse™: Cell Line Development from Design to GMP Master Cell Bank
Driving faster, clinically aligned iPSC cell line development with clean IP
Setting the Standard in Cell Line Development
Pulse™ enables cell therapy innovators to simplify and accelerate the journey from iPSC line to GMP Master Cell Bank — uniting cell line design, reprogramming, gene editing, and GMP master cell banking (MCB) under one integrated platform with transparent, proprietary IP.
Traditional editing approaches can add up to 6 months per edit to development and increase risks to genomic integrity
Cut 12+ months of your timeline
- STAR-CRISPR™ multiplexing enables up to 4 gene edits in a single shot
- Automated high-throughput clonal selection with C.Station & UP.Sight
Licensing complexity and unclear IP hinder path to commercialization.
Integrated IP and licensing
- All IP - including STAR-CRISPR™ and unedited and edited Allo Chassis™ lines - under one roof
Advancing with confidence in a comparatively new regulatory environment.
Regulatory-ready from day one
- Rigorous QC/QA with low-passage banking ensures genomic stability, reproducibility, and monoclonality
- GMP Master Cell Banks, produced in an EMA-certified, FDA/PMDA-compliant facility
Fragmented handoff between Cell Line Development and Manufacturing lead to rework, lost time, and added risk.
End-to-end continuity
- Seamless integration to GMP manufacturing of cell therapy drug product through our Echo™ platform
Traditional editing approaches can add up to 6 months per edit to development and increase risks to genomic integrity
Cut 12+ months of your timeline
- STAR-CRISPR™ multiplexing enables up to 4 gene edits in a single shot
- Automated high-throughput clonal selection with C.Station & UP.Sight
Licensing complexity and unclear IP hinder path to commercialization.
Integrated IP and licensing
- All IP - including STAR-CRISPR™ and unedited and edited Allo Chassis™ lines - under one roof
Advancing with confidence in a comparatively new regulatory environment.
Regulatory-ready from day one
- Rigorous QC/QA with low-passage banking ensures genomic stability, reproducibility, and monoclonality
- GMP Master Cell Banks, produced in an EMA-certified, FDA/PMDA-compliant facility
Fragmented handoff between Cell Line Development and Manufacturing lead to rework, lost time, and added risk.
End-to-end continuity
- Seamless integration to GMP manufacturing of cell therapy drug product through our Echo™ platform
What Pulse™ Delivers
Cut timelines by 12+ months with iPSC cell line deliverables optimized for clinical readiness and manufacturing continuity — here’s what that include:
- Fully characterized, GMP-compliant iPSC Master Cell Bank — ≥200 vials (≥1M cells each), low-passage and genomically stable.
- Comprehensive documentation package — Certificates of Analysis, Certificate of Compliance, traceability logs, clone histories, batch records.
- Pre-validated release assays and QC panel — streamlined handover to GMP manufacturing facility.
- Manufactured in EMA-certified, FDA/PMDA-compliant facility — backed by 15+ years of iPSC and GMP manufacturing experience and 100% program success rate.
- IP under one roof — direct access to STAR-CRISPR™ and Allo Chassis™ iPSC cell lines without third-party licensing
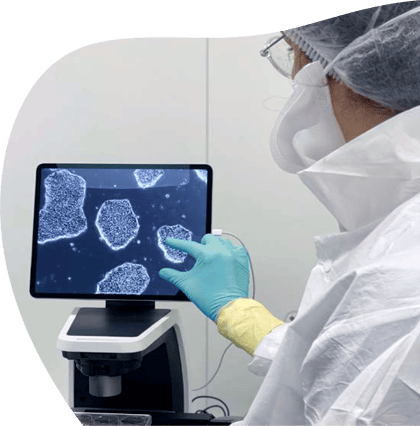
GMP Master Cell Banking
Our turnkey process includes pre-qualified materials, rigorous quality control, genetic stability assessments, and comprehensive GMP documentation, providing the critical proof for your IND.
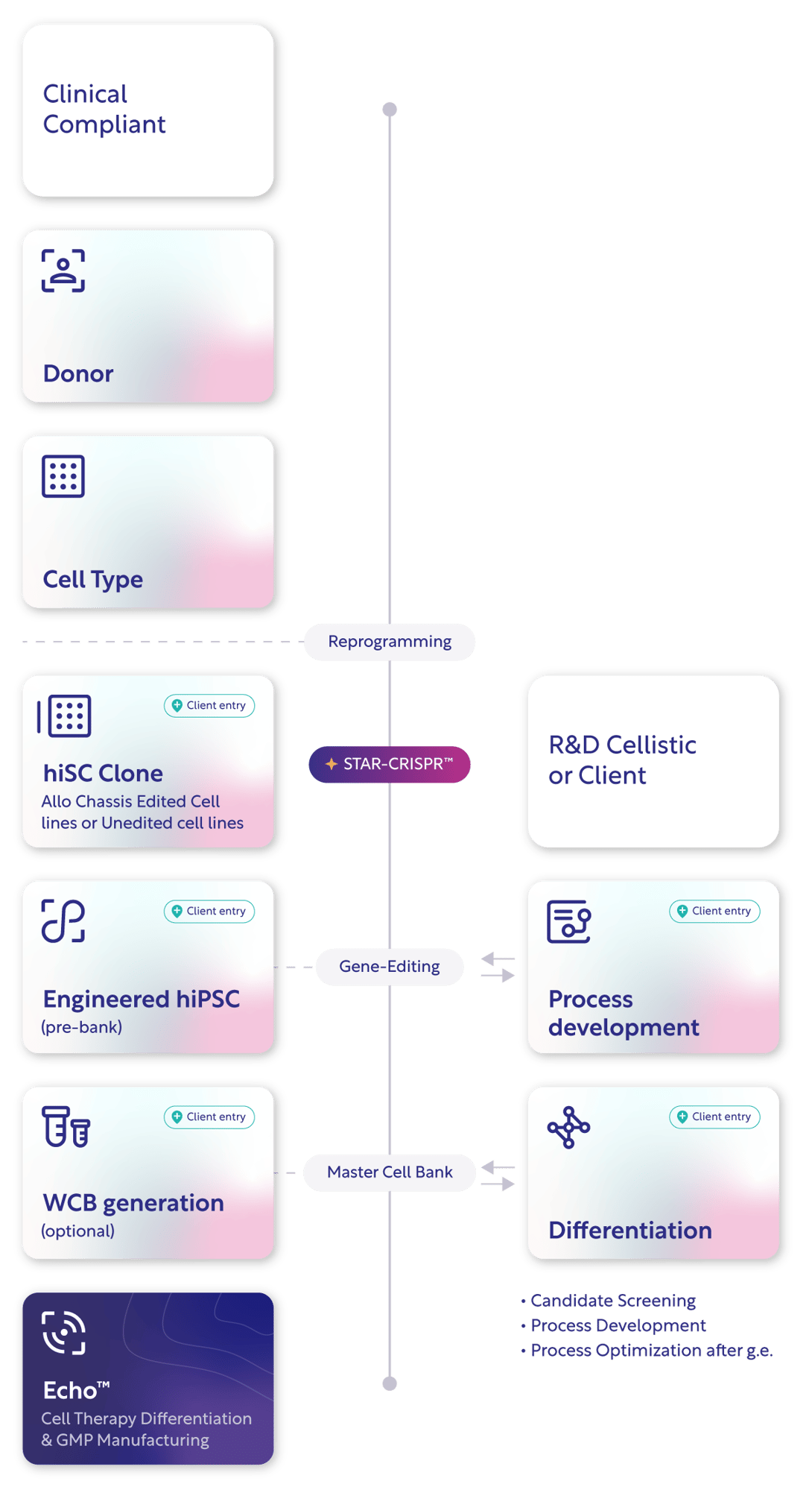
Pulse™: The Integrated Workflow for Clinical-grade iPSCs
Integrating scientific precision with operational scalability — and flexible enough to meet you at any stage of development.
- STAR-CRISPR™ Multiplex editing
Up to four simultaneous edits with high efficiency and minimal off-targets. - Automated Clonal Handling
C.Station & UP.Sight enable high-throughput screening for monoclonality, traceability, and optimal clone selection. - GMP-Aligned Process
Pre-qualified assays, defined materials, and standardized documentation eliminate R&D-to-GMP rework. - All IP under one roof
All core technologies — STAR-CRISPR™, Allo Chassis™, and GMP workflows — fully owned and licensed by Cellistic.
Off-the-shelf iPSC Cell Lines & GMP Master Cell Banks

Why Cellistic?

GMP Manufacturing Excellence
EMA-certified, FDA/PMDA-compliant GMP facility in Mont-Saint-Guibert (Belgium) combining industrialized processes and automation for consistent, clinical-grade manufacturing.
Enabling Technologies
Proprietary platforms — Pulse™ and Echo™, technologies STAR-CRISPR™and Allo Chassis™ — combined with end-to-end CDMO services to accelerate allogeneic iPSC-derived therapy development.
15 Years of iPSC Expertise
With over 15 years of focused iPSC innovation, our scientists have refined differentiation, scale-up, and analytical strategies that define industry benchmarks for consistency, potency, and quality.

Quality & Reliability
Rigorous QC/QA systems and advanced analytical methods ensure consistent, compliant, and high-performing cell therapy materials.
News & Resources
FAQ
Pulse™ is a pre-established, iPSC-derived Cell Line Development platform that streamlines the generation of a GMP Master Cell Bank, providing a consistent, scalable foundation for the entire lifecycle of your cell therapy product.
Yes. Cellistic provides both unedited iPSC lines and gene-edited Allo Chassis™ iPSC lines. We can also generate customer-specific cell lines tailored to your needs.
Yes, we’re flexible. We can generate iPSC lines from your donor material to generate your GMP MCB.
We use automated, closed-system platforms to isolate single iPSCs and expand them into clonal lines. This allows us to select the best-performing clone while ensuring each line is monoclonal, consistent, and reproducible for therapeutic use.
We maintain low passage numbers, use standardized culture conditions, and have rigorous quality controls to ensure iPSC lines remain genetically stable.
Cellistic works exclusively with iPSCs. If you’re considering switching from autologous or donor-derived allogeneic material, we can provide proof-of-concept support to help you explore the transition.
By combining STAR-CRISPR multiplex gene editing with automation, Pulse™ can reduce your cell line development timeline by 12 months or more compared to traditional approaches.
Yes. Cellistic’s facilities are EMA-inspected and certified, and operate in compliance with FDA and PMDA standards.
Yes. We provide comprehensive GMP documentation, including Certificates of Analysis, batch records, and traceability logs.
Our platform offers different entry points and combines proven, validated workflows with flexible steps that can be tailored to your program’s specific needs.
Yes. A license is required for STAR-CRISPR™. For Allo Chassis™, a license is needed if you use our gene-edited cell lines as starting material.
Yes. While Cellistic’s proprietary STAR-CRISPR™ technology is fully owned and integrated into our platform, our team also has experience with other gene-editing technologies and can support their use in your program.
No third-party licenses are required.
Yes, we have in-house and outsourced analytical methods.
Yes, you can tech transfer your own analytical methods. We will work with you to evaluate and implement this as a customized option.
STAR-CRISPR™ combines optimized guide design with high-fidelity nucleases, achieving higher editing efficiency and lower off-target effects than other technologies. All edits are rigorously validated to confirm on-target precision, specificity, and reproducibility.